- The generation and prevention of acid mist in sulfur acid production
Preface
Sulfur acid production facilities are getting larger and larger. Once the acid concentration, acid temperature and moisture control are inaccurate, or the operation is improper, causing equipment corrosion and system leakage, it will lead to an increase in acid mist in the system, resulting in excessive acid mist emissions from the tail gas and air pollution.
I. Analysis of the Causes of Acid Mist Generation during the Production Process
1.1 Impact of Excessive moisture in the drying tower
After the wet air enters the drying tower for drying, if the drying efficiency fails to meet the standard, the dry air containing a small amount of moisture (>0.1g/Nm3) enters the sulfur incineration furnace and, along with the flue gas, enters the converter to react with SO3 and form sulfuric acid vapor. In the flue gas pipeline or the cold and hot heat exchanger, it encounters cold and forms acid mist.
1.2 The impact of corrosion and perforation on the tubes of the hot and cold heat exchanger
The heat exchange tubes of the hot and cold heat exchanger are prone to corrosion by condensing acid, causing short circuits in the converted gas. The flue gas with a high concentration of high sulfur oxide enters the later section, increasing the load on the secondary suction tower. If the leakage is large, the secondary suction tower will not fully absorb SO3, and a large amount of SO3 will be carried into the tail suction system and cannot be completely absorbed, resulting in smoke from the tail gas chimney and excessive acid mist.
1.3 The influence of flue gas temperature changes in the system
During the process of acid production from sulfur, when the flue gas temperature is too high, it will cause the acid temperature to rise, the equilibrium partial pressure of SO3, H2O and H2SO4 vapor on the liquid surface to increase, the driving force to decrease, and the absorption efficiency of SO3 to decrease, resulting in the formation of a large amount of acid mist. Dew point corrosion occurs when the flue gas temperature drops, especially below the dew point of sulfuric acid vapor , because sulfuric acid vapor condenses into condensate acid below its dew point and corrodes equipment.
1.4 The influence of acid absorption temperature
When the absorption acid temperature rises, the equilibrium partial pressure of SO3, H2O and H2SO4 vapors on the liquid surface increases, the absorption driving force decreases, the absorption rate drops, and SO3 and water vapor in the gas phase are more likely to combine to form acid mist. Therefore, high acid temperature will further intensify the generation of acid mist. Therefore, the lower the acid temperature, the higher the absorption rate. However, it is not the case that the lower the acid temperature, the better. For instance, if the acid temperature is too low, it may cause the local temperature to fall below the dew point. Since the reaction between SO3 and water is exothermic, it will release a large amount of heat, leading to an increase in local temperature. But if the overall absorption acid temperature is too low, some areas may still be below the dew point temperature. This results in SO3 not being completely converted into sulfuric acid but forming acid mists. These acid mists will be carried away by the airflow and cannot be effectively absorbed, thereby further reducing the absorption efficiency.
1.5 The influence of absorbed acid concentration
The concentration of the absorbed acid directly affects the absorption efficiency of SO3 and the formation of acid mist. When the acid concentration is too high, the absorption driving force decreases, and SO? in the gas phase is difficult to be absorbed, resulting in an increase in SO? concentration. Moreover, SO? is prone to combine with water vapor to form acid mists. Similarly, when the acid concentration is too low and the water vapor content in the gas phase increases, it leads to incomplete absorption of SO3, which is prone to combine with SO3 to form acid mist.
1.6 Sulfur contains a large amount of organic matter, which causes an increase in the moisture content at the inlet
Sulfur contains a certain amount of organic matter. After the organic matter burns, water is produced. It has been reported [1] that for every 0.1% increase in the mass fraction of organic matter in the raw sulfur, the ρ(H2O) in the furnace gas increases by 0.2575g/m ³. Therefore, it can cause an increase in the amount of imported moisture and acid mist. The calculation formula for acid mist content is:
Parameter description:
[H2O] : Represents the moisture content of the gas after the drying tower (ppm)
K: Represents the process constant (typical value: 0.1-0.5)
α : Represents the slope coefficient (approximately 0.02 to 0.05)
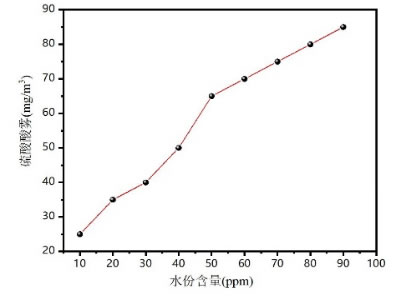
Critical point : When moisture >50 ppm, acid mist rises significantly (exponentially)
The simple graph of acid mist content and moisture content is shown as follows.
II. Control of Acid Mist during the Production Process
2.1 Real-time monitoring of moisture in the drying tower
In order to control moisture in the drying tower, maintain a certain pressure (recommended between 0.02 MPa and 0.05 MPa ) from the drying tower to the sulfur incinerator section to prevent the infiltration of outside wet air, and regularly check the flange and valve sealing of the pipe, with a focus on checking the leakage point from the outlet of the drying tower to the fan section. In addition, an online humidity analyzer is installed at the outlet of the drying tower to adjust the process parameters in a timely manner, etc.
2.2 Prevent corrosion and perforation of hot and cold heat exchange tubes
Choosing materials resistant to sulfuric acid corrosion is the key to preventing the corrosion of heat exchange tubes. For instance, Hastelloy can be selected. This material has excellent corrosion resistance to sulfuric acid and is particularly suitable for high-temperature and high-concentration sulfuric acid environments. In addition, it is necessary to control the formation of condensing acid, increase the temperature of the heat exchange tubes, and ensure that the temperature of the heat exchange tubes is higher than the dew point temperature of the acid to avoid the formation of condensing acid. In addition, condensate acid should be discharged in a timely manner to prevent its accumulation in the heat exchanger and corrosion of equipment, etc.
2.3 Reasonably control the flue gas temperature in the system
Reasonable control of flue gas temperature is the key to reducing the generation of acid mist and equipment corrosion. It is usually necessary to control the flue gas temperature within an appropriate range to avoid it being too high or too low. For instance, at the inlet of the absorption tower, the flue gas temperature should be slightly higher than the dew point temperature of sulfuric acid to prevent dew point corrosion from affecting the service life of the flue, absorption tower and related equipment.
2.4 Control the temperature of the absorbed acid reasonably
To maintain a stable acid temperature and optimize the absorption efficiency, it is necessary to adjust the circulating water volume and water temperature of the acid cooler in the drying and absorption towers, which can ensure that the absorption acid temperature is within a safe range. Generally, the acid temperature of the circulating acid in the drying tower, after passing through the acid cooler, usually does not exceed 60℃. The acid temperatures of the first and second suction towers, after passing through the acid cooler, are usually controlled at around 70℃. Within this temperature range, the formation of acid mist can be effectively reduced.
2.5 Reasonably control the concentration of the absorbed acid
To ensure that SO3 is absorbed quickly, completely and generates less acid mist, it is necessary to frequently control and test the acid concentration to keep it at 98.3%. Because when the acid concentration is greater than 98.3%, the driving force for absorption decreases and the absorption rate decreases. When the acid concentration is less than 98.3%, the absorption process is prone to generating acid mist and white smoke. The lower the concentration, the less complete the absorption of SO3. Therefore, both excessively high and low concentrations will lead to an increase in the equilibrium partial pressure of (PSO3, PH2SO4, PH2O) on the liquid surface, which can easily form acid mists and hinder the absorption process. Only when the acid concentration is 98.3%, the levels of PSO3, PH2SO4 and PH2O on the liquid surface are the lowest and the absorption efficiency is the highest.
2.6 Regularly maintain and inspect the demister
The demister plays a crucial role in the process of sulfuric acid production from sulfur. By regularly inspecting and maintaining the demister, problems existing during its operation can be promptly identified and resolved, ensuring its efficient operation, reducing acid mist, preventing excessive exhaust gas emissions, protecting the environment, and also helping to enhance the stability of the production system and the service life of the equipment.
2.7 Control the quality of sulfur
Table 1 is a comparison table of the influence of organic substances on parameters. In actual production, the moisture content and acidity in sulfur need to be controlled. Excessive moisture will promote the conversion of SO3 into acid mist, while excessive acidity will accelerate the corrosion of equipment.
It is necessary to limit the content of hydrocarbons and other impurities. Incomplete combustion of organic matter will cause carbon black pollution to the system and lead to fluctuations in SO2 in the exhaust gas.
A comparison table of the influence of organic substances on parameters
.jpg)
References:
[1] Tang Guihua, Quality Requirements of Raw Sulfur for Sulfuric Acid Production [J]. Sulfur-phosphorus Design, 1999, 13-17.


